What is Graphene?
Graphene is a versatile carbon-based nanomaterial that is 200x stronger than steel and can stretch up to 25% of its original length. It was discovered by physicists Sir Andre Geim and Sir Konstantin Novoselov, who were awarded The Nobel Prize in 2010 for their research.
- Tightly bonded carbon atoms arranged in a hexagonal lattice
- 200x stronger than steel, yet...
- Like rubber that can stretch up to 25% of its original length
- More electrically conductive than Copper
- Better at conducting heat than any material
- Optically Transparent
- Atomic-level barrier properties
Commercial Applications Across Industries
Cost-Effective and Environmentally Friendly: Incorporating graphene into many materials can reduce their use by 25%+ to decarbonize and reduce Greenhouse Gas emissions.

Industrial
Lubricants, Cement, Chemicals, Composites, Paints & Coatings, Tire & Rubber

Packaging
Beverages, Cosmetics, Food, Labels, Personal Care Products

Consumer
Clothing, Footwear, Styling, LED Lighting

Auto
Tires, Adhesives, Plastic & Foam Parts, Batteries, Wheels, Paint

Sporting Goods
Golf Balls, Field Hockey Sticks, Hydrofoil, Rugby Balls, Bicycle frames

Electronics
Thermally Conductive Adhesives, Batteries, Substrates, Wearable Electronics
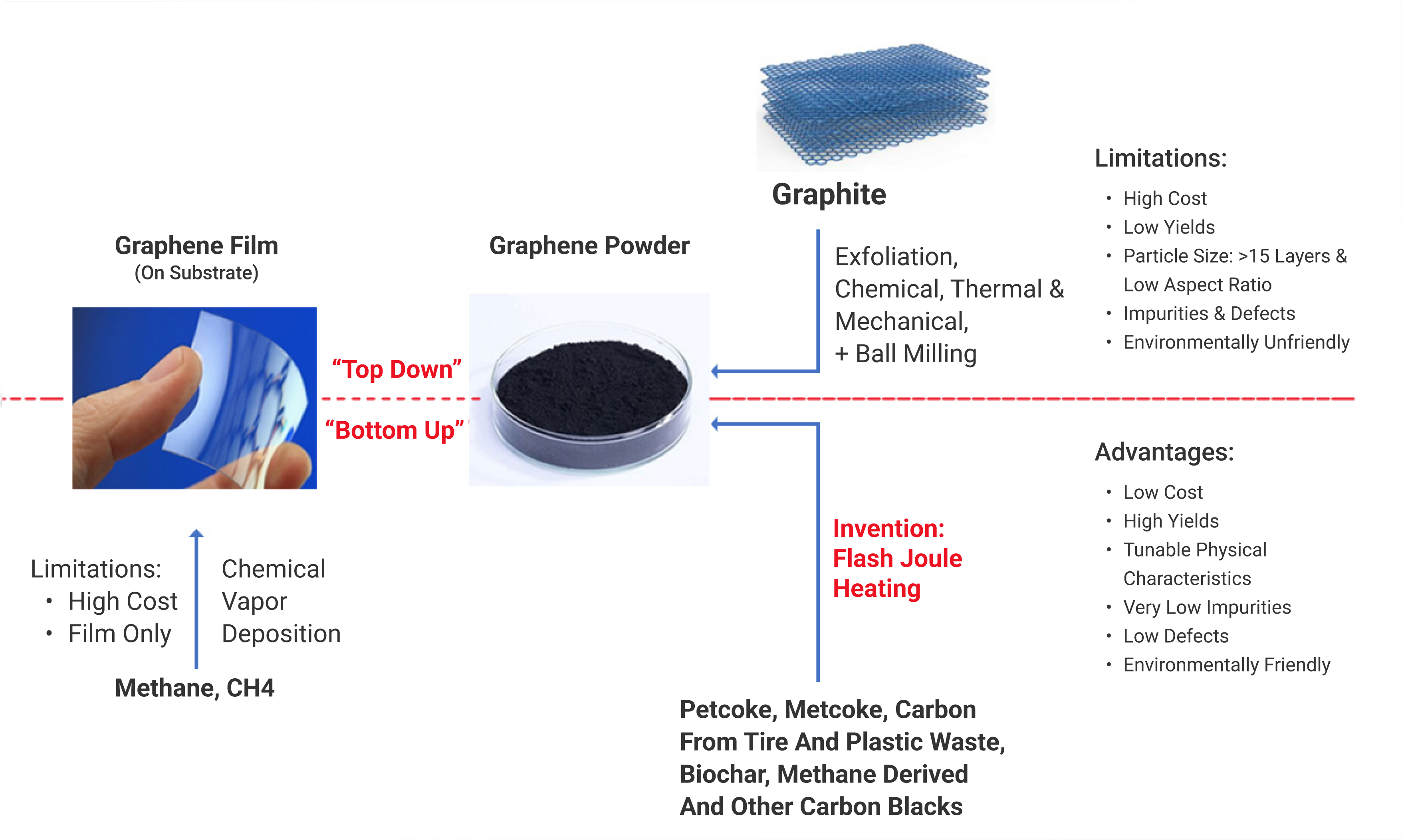
Producing Graphene
Producing high-quality graphene on a large scale at a low cost has been a difficult challenge for the entire industry. Currently, production of mass graphene generally uses relatively expensive graphite as a base material. It requires large amounts of chemical solvents, energy and furnace treatment. This process often results in graphene with a defective perforated structure.